ELEVATION IN BOILING POINT: The boiling point of a solution containing a non volatile solute is always higher than the boiling point of the pure solvent. this increase in boiling point is termed as elevation in boiling point.
CREDIT : GOOGLE
CREDIT : GOOGLE
CREDIT : GOOGLE
CREDIT : GOOGLE
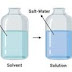
the hydrostatic pressure which develops on account of osmosis is called osmotic pressure or the excess pressure that must be applied on the solution to prevent osmosis is called osmotic pressure.
The solution having same osmotic pressure at a given temperature are called isotonic solution.
if one solution is of lower osmotic pressure ,it is called hypotonic with respect to the more concentration solution. the more concentration solution is said to be hypertonic with respect to the dilute solution.
if one solution is of higher osmotic pressure is applied on the solution the solvent will flow from the solution into the pure solvent through the semipermeable membrane and the process is called reverse osmosis.it is used in desalination of sea water.
ABNORMAL MOLAR MASS: molar masses that are lower or higher than expected values when calculated
(generally using colligative properties) are called abnormal molar masses.
Abnormal molar masses depends upon the total number of moles particles either
after dissociation or association of solute molecules in solvent or solution.
van't hoff factor: The van 't Hoff factor is the ratio between the actual concentration of particles produced when the substance is dissolved and the concentration of a substance ...
CREDIT : GOOGLE

.jpg)


.jpg)



.png)



1 Comments